ISO 13485:2016
MEDICAL DEVICES – QUALITY MANAGEMENT SYSTEM
The medical industry operates under strict regulations and standards to ensure the quality and safety of medical devices. Compliance is crucial, and that’s where the ISO 13485 certification comes into play. Implementing ISO 13485 brings several benefits to organizations. Although it requires initial effort, the resulting quality management system positively impacts operations, sales, and customer satisfaction.
Additionally, the ISO 13485 certificate offers a structured framework to comply with medical device directives, regulations, protocols, and responsibilities. It helps manufacturers fulfill their commitment to producing safe and high-quality medical devices. As an internationally recognized standard of quality and safety for medical device manufacturing, having this ISO certification helps companies be recognized as reputable, trustworthy providers regardless of size or location. It ensures that all medical devices meet regulatory compliance laws and address customer needs, making it the optimal medical device standard in the industry. It is a valuable credential to ensure the safety of professionals and customers in clinics, hospitals, and other medical settings.

ISO 13485:2016 Certification and Auditing Services by CertPro
At CertPro, we recognize the importance of quality management and the advantages of ISO 13485:2016 certification for organizations aiming to improve their medical device manufacturing processes. We provide comprehensive assistance to companies pursuing ISO 13485:2016 certification. Our team of skilled experts will support you every step of the way, ensuring that your organization’s practices align with the latest ISO standards. We will work closely with your team to develop and implement a customized QMS (Quality Management System) that meets your unique needs and complies with industry standards for medical device quality.
Why choose CertPro for ISO 13485:2016 certification and auditing?
CertPro is a trusted and dependable ally for ISO 13485:2016 certification and auditing services. With almost a decade of expertise in the industry, we possess a profound comprehension of the intricacies associated with medical device manufacturing. Here are several compelling reasons why CertPro is the ideal selection for your ISO 13485:2016 certification requirements:
| Factors | CertPro Advantage |
|---|---|
| Time to Certification | 4x faster than traditional approaches |
| Price | Competitive rates with flexible options |
| Process | Streamlined and efficient methodology |
| Expertise | 10+ years of industry experience |
CertPro’s Cost-Effective Approach to ISO 13485:2016 Certification
When considering ISO 13485:2016 certification, organizations may find the total cost overwhelming, as it includes various factors that influence overall expenses. At CertPro, we understand the importance of cost-effectiveness and are focused on providing customized, budget-friendly solutions tailored to your organization’s needs. Here’s an overview of our cost-effective approach to ISO 13485:2016 certification:
| No. of employees | Timeline | Cost (approx.) |
| 1 – 25 | 6 weeks | 4750 USD |
| 25-100 | 8 weeks | 6750 USD |
| 100-250 | 8-10 weeks | 9750 USD |
| 250 plus | 12 weeks | Custom plans |
Overview of ISO 13485: 2016 Understanding the International Standard for Medical Devices
ISO 13485 is an ISO standard that establishes the expectations and requirements for a medical device quality management system within the medical device industry. Developed based on ISO 9001 and incorporating all the fundamental principles of the ISO Quality Management System and other similar management systems, it enables medical device manufacturers to establish and maintain effective processes. Its primary objective is to harmonize medical device regulatory requirements. It encompasses specific mandates for the manufacturing, installation, and servicing of medical devices, emphasizing:
- Implementing a Quality Management System with several enhancements
- Adopting a risk management approach to product development and realization
- Validating processes to ensure effectiveness
- Complying with statutory and regulatory requirements.
- Establishing efficient product traceability and recall systems
By adhering to ISO 13485, companies can reduce safety and legal risks while creating more efficient work environments. This standard for quality and safety in medical device manufacturing enhances a company’s reputation and trustworthiness. The latest version of ISO 13485 undergoes review and revision every five years to address evolving requirements and industry needs.
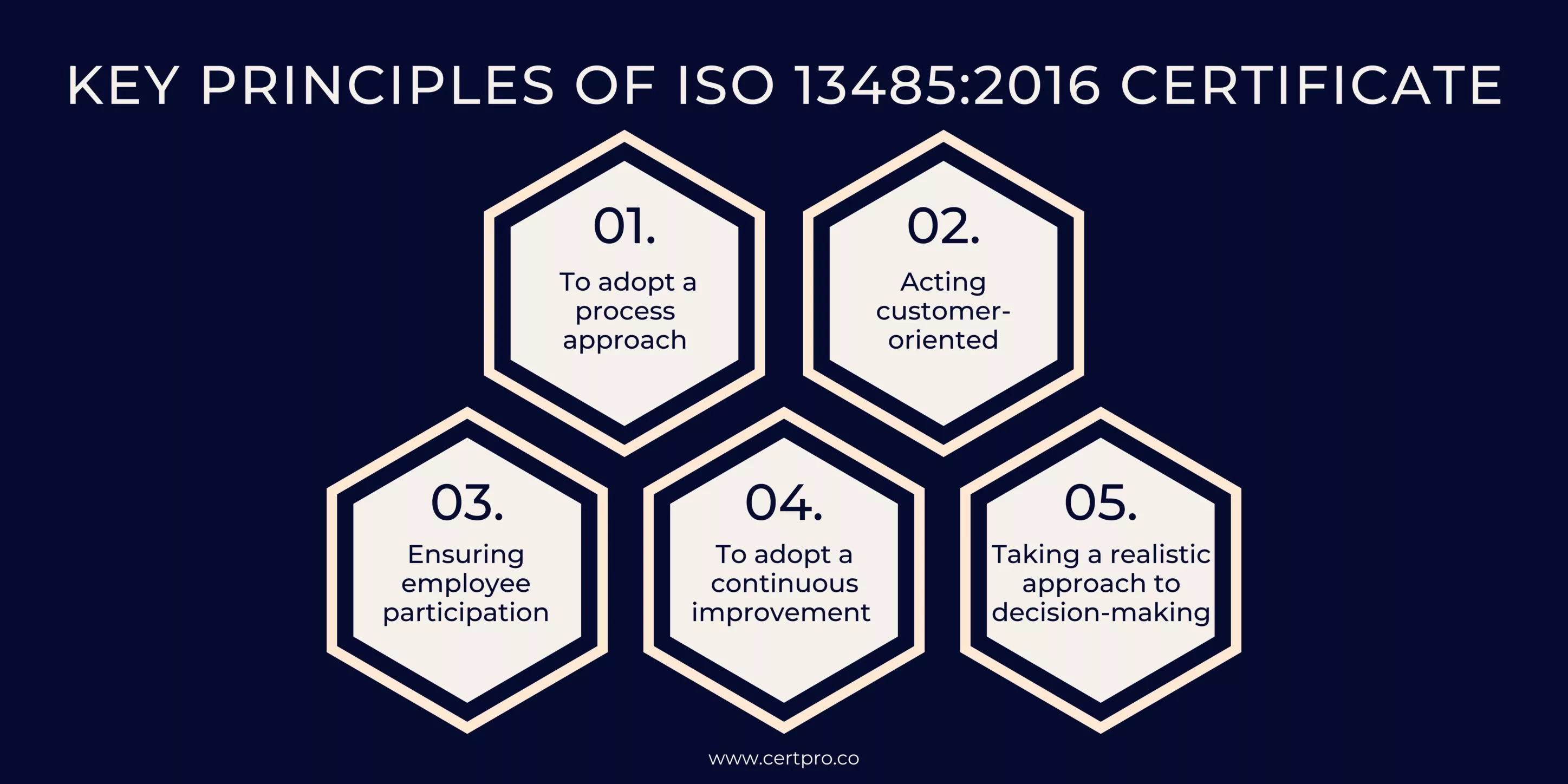
KEY PRINCIPLES OF ISO 13485:2016 CERTIFICATE
The basic principles of the ISO 13485 Medical Device Quality Management System are as follows:
- To adopt a process approach
The process approach expects all personnel to operate under specified processes in their responsibilities. It includes adhering to procedures, job descriptions, workflows, and application instructions outlined in the standard. Efficient resource utilization is a key focus. It enables timely recognition of potential risks, facilitates prompt response to hazards, and allows for proactive measures to avoid unforeseen situations.
- Acting customer-oriented
Businesses that value the customer at their center always stay one step ahead. Meeting customer needs and expectations becomes essential. Giving importance to customers is necessary to create customer satisfaction, build reliance, gain a respectable and prestigious position in the market, and take the lead in the competition with rivals.
- Ensuring employee participation
This principle of ensuring employee participation is crucial, emphasizing the engagement and awareness-raising of all employees. It is a fundamental principle applicable to all management systems. Ineffective teamwork and a lack of ownership and responsibility for problem-solving arise when employees are not actively involved. Conversely, employees should take responsibility for their performance. Then employees view themselves as integral to the organization and become more efficient and proactive.
- To adopt a continuous improvement approach
This principle is essential for ensuring the ongoing performance of the enterprise. Every employee within the company should strive for the regular improvement of services, processes, or systems. Moreover, the organization cannot respond promptly and flexibly to the opportunities without this commitment. The ISO 13485 Medical Devices Quality Management System standard recognizes this principle as fundamental and aligns it with other quality management systems.
- Taking a realistic approach to decision-making
Enterprises should make data-based and rational decisions to avoid unpredictable situations. An organization must utilize and analyze all the data obtained from its activities to make accurate and effective decisions.
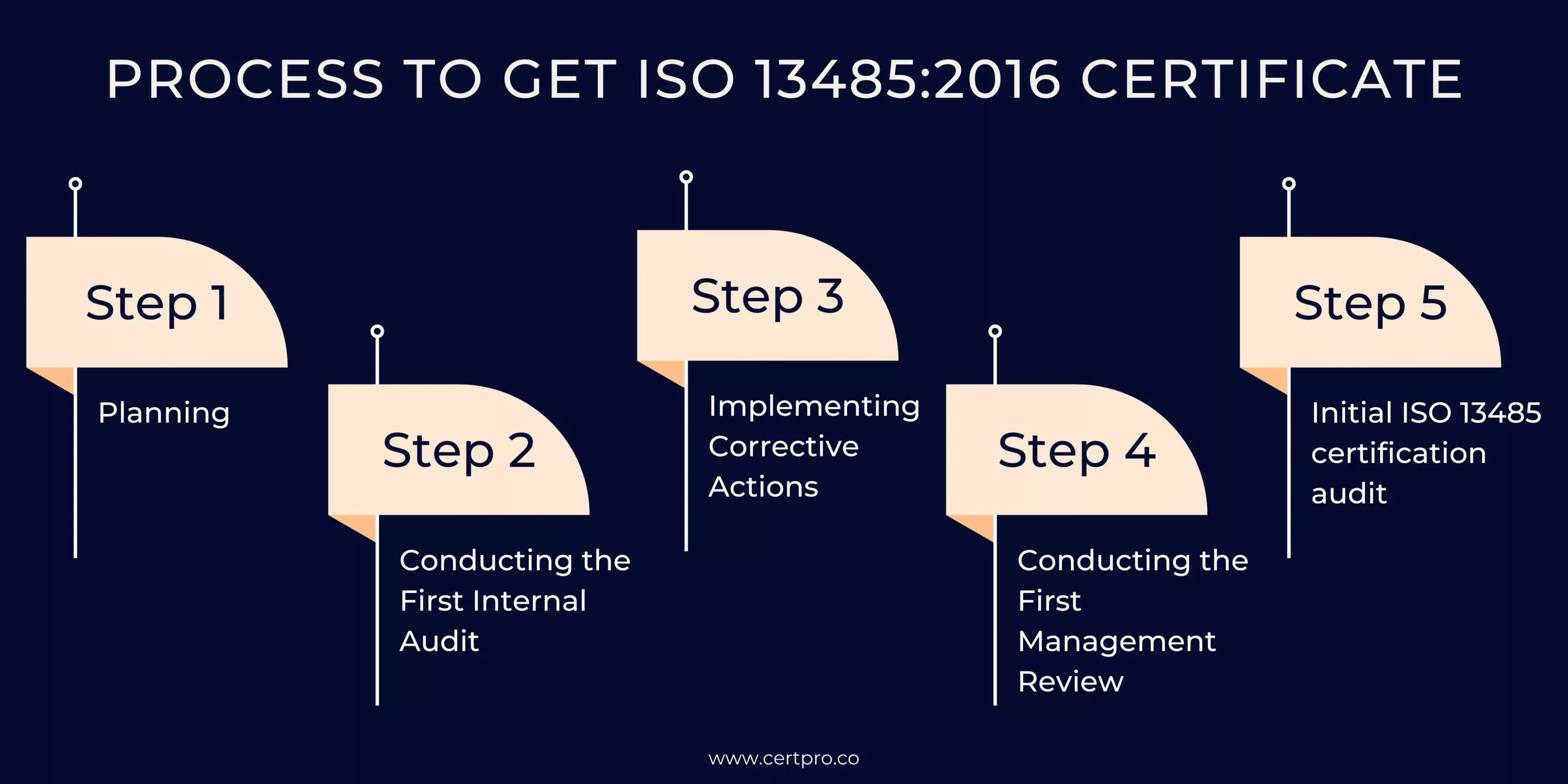
PROCESS TO GET ISO 13485:2016 CERTIFICATE
While the initial requirements and steps to obtain ISO 13485 certification may vary depending on the business or organization, the following steps should help you get started:
Step 1: Planning
At this stage, an organization develops a plan for establishing a Quality Management System (QMS) that aligns with the quality planning requirements of the ISO 13485 standard. It includes creating a quality manual and implementing an optimized method for documenting quality plans when making changes to the QMS. In addition, the organization has to complete the following tasks:
- Obtain the ISO 13485 standard and related documents.
- Identify and establish relevant processes.
- Assign internal auditors and teams to oversee these processes.
- Create a schedule, define workflows, and establish guidelines for each process.
- Conducting gap analysis for each process or task.
- Providing ISO 13485 certification training for process owners
- Enhance and finalize processes.
- Standardize processes and document necessary records.
Step 2: Conducting the First Internal Audit
Conduct the first internal audit to verify the effectiveness of your QMS before the initial audit by the third-party certification body. It enables the organization to identify nonconformities, determine their root causes, and develop appropriate actions to mitigate and resolve them.
Step 3: Implementing Corrective Actions
After reviewing the internal audit report, you can identify any issues or areas that require elimination or improvement. Initiate a Corrective and Preventive Action (CAPA) process to address these. This quality management procedure enables everyone involved in those areas to implement necessary actions and process improvements.
Step 4: Conducting the First Management Review
It is necessary to have the top management review the organization’s quality objectives before the third-party audit. After the Management review, the management concludes by finalizing a list of action items, and changes are made based on internal audit findings. This list serves as a guide for the subsequent management review before applying for certification.
Step 5: Initial ISO 13485 certification audit
In this step, your organization initiates the process by researching and selecting a recognized ISO 13485 certification body to conduct the certification audit. The application for certification begins, including filling out and reviewing the form before submission to the certification body. Contract signing may also occur during this stage. This step consists of two stages:
Stage 1: Documentation Survey: The third-party auditor focuses on assessing the organization’s documentation of systems, ensuring the presence of the CAPA process, management review schedule, and overall QMS. The preparation of various documents is necessary, including the quality manual, organizational chart, internal auditing process and schedule, CAPA protocols and log, and management review findings and process.
Stage 2: Primary Audit: This stage verifies that the organization satisfies all the ISO 13485 certification requirements, including compliance with international market regulatory requirements. The auditor evaluates the effectiveness of your quality system by sampling records from each implemented process. Additionally, this stage determines the schedule for surveillance audits (typically conducted annually) and reassessments.
THE ISO 13485:2016 REQUIREMENTS
The ISO 13485 standard contains eight sections (clauses). The first three clauses explain the scope, reference, definition, and other general information. The last five clauses are as follows:
Clause 4: Quality Management System
This clause targets two specific aspects of a Quality Management System: General Requirements and Documentation Requirements.
General Requirements: The general requirements dictate that organizations implement a quality management system, adhere to the standard, and maintain written procedures for documentation and risk management, ensuring compliance with those procedures.
Documentation Requirements: The ISO 13485 documentation requirements mandate that medical device manufacturers create a quality manual or equivalent, maintain product specifications and guidance, implement a document control plan, and establish a record control plan for data integrity and security.
Clause 5: Management Responsibility
The Management team of an organization implementing ISO 13485 must demonstrate specific responsibilities. They focus on the end user’s needs, ensure compliance with rules during manufacturing, communicate the importance of quality policies and procedures to the employees, delegate authority, and perform periodic reviews for system improvements like the Management Review.
Clause 6: Resource Management
The top management must provide the essential resources to ensure compliance with ISO 13485 in the organization. They must support the quality system throughout the organization by assigning proper personnel, infrastructure, tools, and equipment; succession planning; and risk aversion planning. Simply implementing a quality system is not sufficient.
Clause 7: Product Realization
The development of a new product encompasses the entire process, from conceptualization to design and implementation. ISO 13485 emphasizes the significance of communication and procedures throughout the product life cycle. A robust quality system ensures that an organization captures initial ideas and requirements, plans and develops the product, and monitors customer use through well-defined processes.
Clause 8: Measurement, Analysis, and Improvement
ISO 13485 also emphasizes the significance of tracking customer feedback and monitoring and measuring product performance once it is released. Achieving this involves managing complaints, making necessary notifications and reports to regulatory authorities, identifying and addressing nonconforming products, and continuously monitoring product performance while striving to improve processes.
BENEFITS OF ISO 13485:2016 CERTIFICATION
Implementing ISO 13485 in a medical device manufacturing business offers several key benefits, including:
- Enhanced customer satisfaction: Achieve customer requirements consistently by providing reliable products and services that meet their expectations.
- Cost savings: Continuously improving processes leads to increased operational efficiency, resulting in reduced operating costs for your organization.
- Strengthened stakeholder relationships: Improve the perception of your organization among staff, customers, and suppliers, promoting robust and more trusting relationships.
- Compliance with legal regulations: Gain a comprehensive understanding of statutory and regulatory requirements, ensuring compliance and minimizing legal risks for your organization and customers.
- Established business credibility: Independent verification against the globally recognized ISO 13485 standard establishes your organization’s credibility and demonstrates your commitment to quality.
- Competitive advantage in regulated sectors: Certification provides a competitive edge, particularly in highly regulated sectors, where procurement specifications often require ISO 13485 certification as a prerequisite for supply.
- Enhanced risk management: With improved product consistency and traceability, potential issues can be identified and rectified more effectively, minimizing risks and enhancing overall risk management capabilities

ISO 13485:2016 CERTIFICATION COST
ISO 13485 certification costs depend on ISO 13485 accreditation bodies, which consider several factors:
- They consider the size of the organization.
- They assess the number of certified locations required.
- The cost varies based on the products and services provided by the company.
- The number of employees into account.
- They determine the number of principles that need to be protected.
- The cost also considers the risks associated with the company.
Accreditation bodies consider these factors in determining the cost of ISO 13485 certification for organizations seeking to comply with the standard.
CHALLENGES AND SOLUTIONS IN ISO 13485:2016 CERTIFICATION FOR MEDICAL DEVICE MANUFACTURING
The medical device manufacturing industry faces six significant challenges that require attention and resolution.
- Neglecting compliance with medical device manufacturing regulations
Ensure thorough understanding and compliance with applicable rules to meet safety and quality requirements.
- Insufficient understanding of relevant standards
Acquire in-depth knowledge of ISO 13485 requirements and stay updated with industry standards through training and continuous learning.
- Avoidance of unapproved components, methods, and facilities
Implement robust supplier qualification processes, verify component traceability, and use approved methods and facilities for manufacturing.
- Ensuring security measures are in place
Establish strict data security measures, safeguard intellectual property, and implement access controls to protect sensitive information.
- Managing the high cost of production
Optimize manufacturing processes, explore cost-effective sourcing options, and streamline operations to reduce production expenses.
- Failure to utilize external expertise effectively
Engage qualified consultants or auditors with expertise in ISO 13485 to provide guidance, conduct assessments, and assist in certification preparation.
Validity Period of ISO 13485:2016 Certification: Certification Duration and Renewal
After obtaining the certification, the ISO 13485 certificate remains valid for three years. During this period, the organization undergoes annual surveillance audits to verify its adherence to ISO quality standards. These audits ensure ongoing compliance and the effective functioning of the quality management system. The surveillance audits thoroughly evaluate whether the organization meets the requirements specified in ISO 13485. This periodic assessment demonstrates the organization’s unwavering commitment to maintaining the quality standards established by the certification. The organization undergoes a recertification audit upon completing the three-year certification period to assess its ongoing compliance and eligibility for the ISO 13485 certification. ISO 13485 certificate verification is essential to ensuring continual compliance and quality assurance within the organization.
ISO 13485:2016 CERTIFICATION Simplicity: PARTNERING WITH CERTPRO FOR SUCCESS
CertPro specializes in assisting businesses to obtain ISO 13485 certification through their comprehensive auditing, consulting, and certification services. With a team of experienced auditors and consultants, CertPro guides your business through the certification process, adhering to industry best practices and international standards. They assess your quality management system for medical devices, identify areas for improvement, and help implement effective processes and controls. CertPro provides documentation support and expert guidance to ensure compliance with ISO 13485 requirements. By partnering with CertPro, your business can demonstrate its commitment to quality and safety in the medical device industry, enhance customer confidence, and meet regulatory obligations. Achieving ISO 13485 certification with CertPro’s assistance strengthens your reputation, increases market opportunities, and facilitates access to global markets for your medical devices.
FAQ’s
HOW LONG DOES IT TAKE TO OBTAIN ISO 13485:2016 CERTIFICATION?
The time required for ISO 13485 certification depends on various factors, including the organization’s readiness and the complexity of its quality management system. Typically, it can take several months to implement the necessary processes, undergo audits, and address any non-conformities identified.
WHAT HAPPENS After OBTAINING ISO 13485:2016 CERTIFICATION?
After achieving ISO 13485 certification, the organization should maintain and improve its quality management system. It involves conducting regular internal audits, addressing non-conformities, monitoring customer feedback, and participating in surveillance audits by the certification body.
IS ISO 13485 CERTIFICATION MANDATORY FOR MEDICAL DEVICE MANUFACTURERS?
ISO 13485 certification is not required in all countries. However, it is well-known and frequently required by regulatory bodies and clients in the medical device industry as proof of compliance with quality standards.
CAN ISO 9001 CERTIFICATION BE USED AS A SUBSTITUTE FOR ISO 13485 CERTIFICATION?
While ISO 9001 and ISO 13485 share similarities, ISO 9001 focuses on general quality management principles, while ISO 13485 specifically addresses the requirements for medical device manufacturing. Depending on the regulatory and customer requirements, ISO 9001 certification may not be sufficient, and ISO 13485 certification may be necessary.
WHAT IS THE ROLE OF A CERTIFICATION BODY IN THE ISO 13485 CERTIFICATION PROCESS?
A certification body is an independent organization that assesses an organization’s compliance with ISO 13485. They conduct audits, review documentation, and issue the ISO 13485 certificate if all requirements are satisfied. It is necessary to choose a reputable and accredited certification body.
UNDERSTANDING ISO 42001: A GUIDE FOR RESPONSIBLE AI MANAGEMENT SYSTEMS
The invention of artificial intelligence (AI) has changed the operational processes of many industries. However, the rapid growth of technology increases ethical, security, and privacy-related concerns. Therefore, the International Organization for Standardization...
EUROPEAN UNION’S ARTIFICIAL INTELLIGENCE ACT: HOW THIS GROUNDBREAKING LAW AFFECTS YOUR BUSINESS
Nowadays, Artificial Intelligence (AI) is transforming our lives exceptionally well. AI is now streamlining healthcare services, providing virtual assistance, and fulfilling queries. Technologies have boons and curses. Similarly, AI creates many concerns about...
How to Implement GRC Frameworks in 2024: Step-by-Step Guide
The rapidly evolving business environment, complexity, and accountability enhance the importance of the organization's governance, risk management, and compliance initiatives. Therefore, if your company finds difficulties expanding, recheck your organization's...