ISO 17025:2017
TESTING AND CALIBRATION LABORATORIES
ISO 17025:2017 certification refers to the recognition granted to laboratories that have demonstrated compliance with the requirements specified in the standard. It signifies that the laboratory possesses the necessary competence, impartiality, and consistency in conducting testing and calibration activities. It applies to all organizations with laboratory activities, regardless of size.
There has been a significant rise in the importance and need for the certification. Certification to the ISO 17025 standard ensures that a laboratory possesses the required technical expertise, resources, and processes to produce reliable and accurate test results. It enhances the organization’s credibility, reputation, and customer confidence. Industries like pharmaceuticals, food processors, and forensic sciences, where precise and reliable measurements are crucial, are particularly interested in ISO 17025 certification.
Obtaining the certification offers several benefits for organizations. It improves operational efficiency, reduces errors, and enhances the overall quality of testing and calibration processes. Accredited laboratories gain recognition in domestic and international markets, facilitating acceptance of their test results. The certification also assists organizations in meeting regulatory requirements and supports continuous improvement initiatives.

Certification and Auditing Services by CertPro
At CertPro, we understand the significance of quality management and the benefits of ISO 17025:2017 certification for organizations seeking to enhance their testing and calibration capabilities. We offer extensive support to organizations pursuing ISO 17025:2017 certification. Our team of experienced professionals will guide you throughout the certification process, ensuring that your laboratory’s practices align with the latest ISO standards. We will collaborate closely with your team to develop and implement a tailored QMS (Quality Management System) that meets your specific requirements and industry standards.
Why choose CertPro for ISO 17025:2017 certification and auditing?
CertPro is a reputable and reliable partner for ISO 17025:2017 certification and auditing services. With our extensive experience of nearly ten years in the field, we have developed a deep understanding of the complexities involved in testing and calibration management. Here are some compelling reasons why CertPro is the right choice for your ISO 17025:2017 certification needs:
| Factors | CertPro Advantage |
|---|---|
| Time to Certification | 4x faster than traditional approaches |
| Price | Competitive rates with flexible options |
| Process | Streamlined and efficient methodology |
| Expertise | 10+ years of industry experience |
CertPro’s Cost-Effective Approach to ISO 17025:2017 Certification
| No. of employees | Timeline | Cost (approx.) |
| 1 – 25 | 6 weeks | 4750 USD |
| 25-100 | 8 weeks | 6750 USD |
| 100-250 | 8-10 weeks | 9750 USD |
| 250 plus | 12 weeks | Custom plans |
UNDERSTANDING ISO 17025:2017 STANDARD
ISO 17025 is the standalone certification standard that establishes the fundamental criteria for ensuring the competency and quality management system (QMS) of testing and calibration laboratories. It provides a framework to ensure that laboratories consistently produce reliable and accurate results. The purpose of ISO 17025 is to establish a framework for laboratories to demonstrate their technical competency, impartiality, and ability to produce accurate and reliable test results. It aims to ensure that laboratories consistently meet defined quality standards, enhancing confidence in their capabilities and promoting international acceptance of their test results.
ISO 17025 applies to laboratories across various sectors, including government-owned, industry-operated, and other organizations. It is advantageous for universities, research centers, governments, regulators, inspection bodies, product certification organizations, and other conformity assessment bodies that conduct testing, sampling, or calibration activities. The standard covers various aspects such as management system requirements, technical competence, equipment and measurement traceability, handling of test items, and reporting results. The standard provides guidelines for establishing and maintaining a quality management system specific to the laboratory’s needs.
By adhering to ISO 17025, laboratories can demonstrate their technical competence, ability to deliver reliable results, and compliance with internationally accepted practices. It helps build confidence in their services among customers, regulatory bodies, and accreditation authorities. The standard also promotes effective quality management systems, risk assessment, and continuous improvement within laboratories, ensuring the validity and reliability of their testing and calibration processes.
KEY PRINCIPLES OF ISO 17025:2017 CERTIFICATION
The principles underlying ISO 17025 certification are:
- Capacity: Laboratories must possess the necessary resources, including skilled personnel, suitable facilities and equipment, quality control measures, and established procedures. These resources are essential for laboratories to effectively conduct their work and generate technically valid and reliable results.
- Exercise of Responsibility: This entails allocating authority to individuals within the laboratory organization to carry out specific functions within the defined scope of work. The organization must demonstrate accountability for the outcomes and results of its work, ensuring that responsibilities are clearly defined and executed with diligence and integrity.
- Scientific Method: Emphasizes that laboratories should adhere to accepted scientific approaches, preferably those that have achieved consensus in the field. Any deviations from these accepted approaches must be supported and justified in a manner that is generally acceptable to experts in the relevant field. It makes sure that the laboratory’s work maintains the highest level of credibility and reliability and adheres to strict scientific practices.
- The Objectivity of Results: Laboratories mostly base their results on measurable or derived quantities. Subjective test results should only be generated by qualified individuals and identified as Subjective or known to be mainly Subjective by experts in the relevant testing field.
- Impartiality of Conduct: Persons performing laboratory tests and calibrations should prioritize their pursuit of technically valid results using accepted scientific approaches, with all other influences being considered secondary and not allowed to take precedence.
- Traceability of Measurement: Laboratories establish an unbroken chain of comparison between recognized measurement systems and their own devices, ensuring accurate and reliable results throughout the measurement process, including uncertainty assessment.
- Repeatability of Test: It states that subsequent testing, utilizing the same procedures, equipment, and personnel as the previous execution of the test, will yield the same results within acceptable deviations. The test should consistently produce objective results that remain consistent over time.
- Transparency of Process: Laboratory processes that produce objective results should be subject to internal and external scrutiny. It enables the identification and mitigation of factors that may potentially hinder the laboratory’s ability to generate technically valid results that are objective and based on scientific methods.
While these eight principles may not encompass every aspect of each requirement in the standard, they provide a broad understanding for laboratory personnel regarding the rationale behind most individual conditions. They also empower assessors to exercise their professional judgment when evaluating a laboratory’s adherence to each requirement within the standard.

STEPS TO OBTAIN ISO 17025:2017 CERTIFICATION
ISO 17025 certification is crucial for laboratories to demonstrate their competence and reliability in testing and calibration. Follow these steps to ensure compliance and gain recognition for meeting international quality standards.
Step 1: Awareness and Understanding of the Standard:
Obtain knowledge of ISO 17025 terminology and requirements. Conduct training sessions to prepare employees for implementing the Quality Management System (QMS) and ensure awareness of new practices throughout the laboratory
Step 2: Formulation of Quality Policy
Formulate a clear and concise quality policy to establish the foundation of your laboratory’s Quality Management System (QMS). Ensure the policy aligns with ISO 17025 requirements and encompasses objectives such as continual improvement, employee engagement, and management reviews. Use analytical tools like the SMART (Specific, Measurable, Achievable, relevant, and Time-bound) model to develop a policy tailored to your laboratory’s context.
Step 3: Conduct a Formal Gap analysis.
Perform a comprehensive gap analysis to determine your laboratory’s operations and Quality Management System (QMS) compliance with the ISO standard. Identify areas of non-compliance with ISO requirements, allowing you to gauge the level of compliance. This analysis will enable you to identify the necessary improvements in your operations and QMS to bridge the gaps and achieve compliance.
Step 4: Document Preparation:
Thoroughly prepare the documentation for your Quality Management System (QMS) and laboratory operations. Make sure all documents are accurate and up-to-date. After having them checked by external auditors from the certification body. Key documents to prepare include the quality manual, functional procedures, system procedures, system formats, test procedures, work instructions, and validation methods.
Step 5: Implement the QMS:
Utilize the documented procedures and work instructions to implement the QMS. Assign responsibilities to staff members and obtain the necessary resource support from management. Create a comprehensive checklist of steps to ensure a smooth implementation process and adherence to QMS requirements to maintain a strong level of compliance.
Step 6: Conduct an Internal Audit
Conduct a thorough internal audit to assess the alignment of your operations and QMS with the ISO standard. Engage experienced assessors from your team or external agencies with in-depth knowledge of the ISO laboratory standard. The audit will identify any non-conformities and provide an audit report, enabling you to implement necessary changes and corrective measures to eliminate non-conformities and qualify your lab for ISO accreditation.
Step 7: Application for Certification:
Search for registered certification bodies and choose one that aligns with your preferences. Apply and receive a price quote and an estimated timeframe for the certification assessment. If the terms are acceptable to you, proceed with the certification process.
Step 8: Obtain the Certification
The certification body you have selected will perform a comprehensive on-site assessment of your operations, quality management system (QMS), documentation, and employee responsibilities. They will interview the employees, observe selected test procedures, or inspect specific calibration equipment. After concluding the assessment, the certification body will verify your laboratory’s adherence to the ISO standard if no non-conformities are detected. Subsequently, they will grant the certification.
They will notify you after identifying non-conformities or discrepancies. You must rectify them within a specified timeframe to obtain the certification.

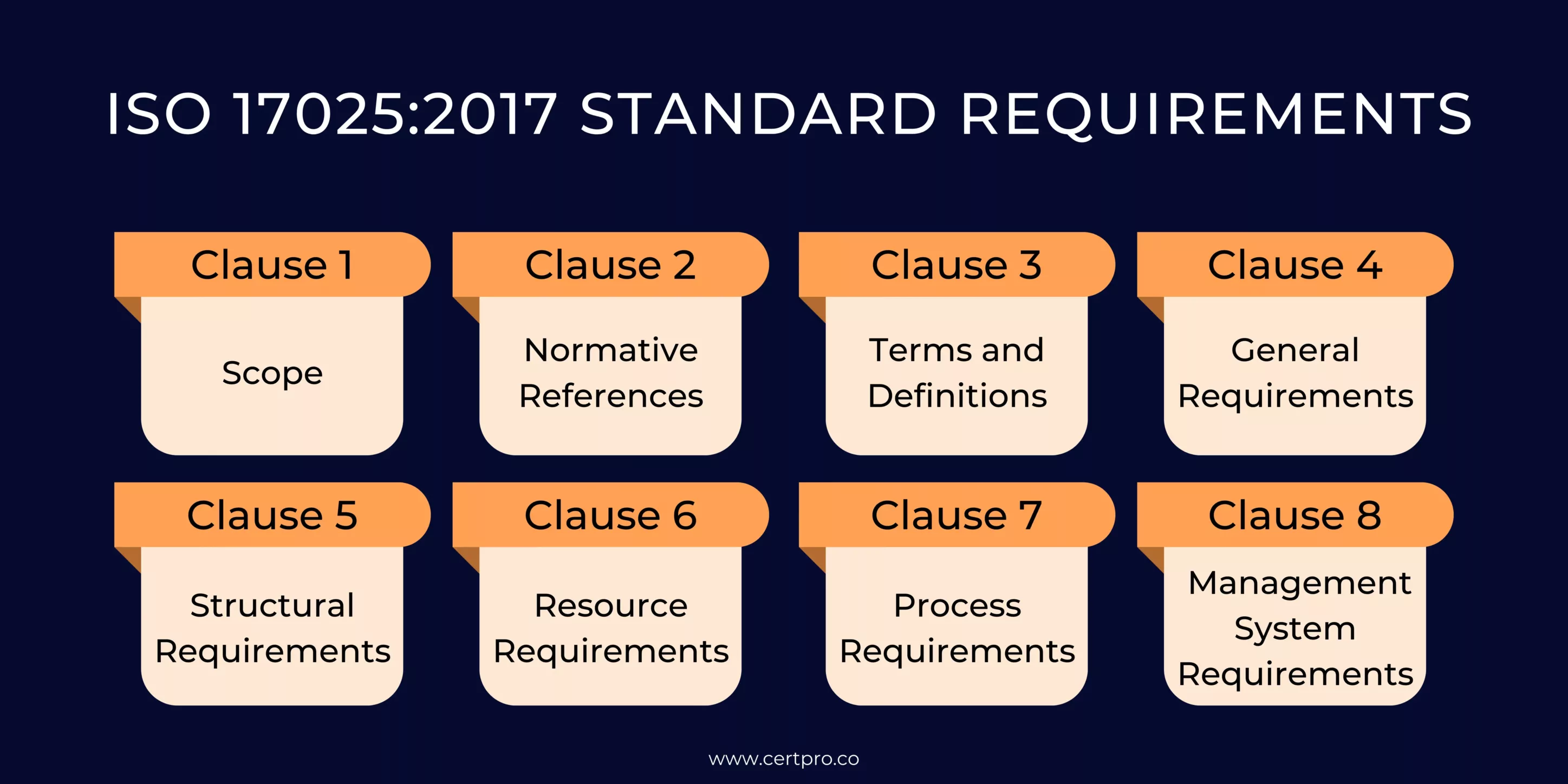
ISO 17025:2017 STANDARD REQUIREMENTS
Organizations are required to be aware of the numerous ISO 17025 standard requirements to implement the essential compliance protocols. The 2017 version of ISO 17025 introduces notable changes, replacing the Management Requirements. Requirements clauses with General Requirements and Structural Requirements It adds clauses 6 to 8, detailing Resource, Process, and Management System Requirements. This version specifies the general requirements for guiding laboratories in ensuring their competence and impartiality.
The ISO 17025 standard consists of the following clauses and elements:
Clause 1. Scope:
Discuss the objective of the standard, its applicability, and the purpose of ISO 17025.
Clause 2. Normative References:
Provide brief details on how specific guides and standards are referenced within the requirements to ensure consistent implementation and compliance.
Clause 3. Terms and Definitions:
Defines the terminology used throughout the standards.
Clause 4: General Requirements
It addresses the two primary requirements of the standard: impartiality and confidentiality. It ensures that laboratories maintain their independence and integrity in producing accurate testing results while safeguarding the confidentiality of sensitive information.
Clause 5: Structural Requirements
It encompasses the laboratory’s organizational structure, components, processes, and adherence to an efficient management system. These requirements ensure effective and well-organized laboratory operations.
Clause 6: Resource Requirements
It outlines the essential components required by a laboratory. The components encompassed in this clause consist of general requirements, facilities and environmental conditions, personnel, metrological traceability, equipment, and externally provided products and services. Adhering to these resource requirements ensures the laboratory has the necessary resources to perform accurate and reliable testing and calibration activities.
Clause 7: Process Requirements
It outlines the 11 key processes that laboratories need to follow to improve and implement the efficiency of the standard’s requirements. These processes include reviewing requests, tenders, and contracts; selecting, verifying, and validating methods; conducting sampling; handling test or calibration items; maintaining technical records; evaluating measurement uncertainty; ensuring result validity; reporting results; managing complaints; addressing nonconforming work; and controlling data and information management. Adhering to these process requirements ensures that laboratories operate consistently and effectively, leading to reliable and accurate test and calibration results.
Clause 8: Management System Requirements
It highlights the two options available for organizations to comply with the standard.
Option A: This applies to laboratories with a separate Quality Management System (QMS).
Option B: This applies to laboratories that are part of larger organizations or have their own established management systems aligned with ISO 9001:2015.
The clause covers various activities within the management system, including management system documentation, Control of management system documents, Control of records, actions to address risks and opportunities, improvement processes, corrective actions, internal audits, and management reviews. These requirements ensure that laboratories have management systems to support the implementation and maintenance of ISO 17025, leading to consistent and reliable testing and calibration practices.
BENEFITS OF ISO 17025:2017 CERTIFICATION
By achieving ISO 17025 accreditation, your laboratory will gain the following benefits:
- Reputation boost: ISO 17025 is a recognized standard that enhances credibility and reliability in laboratory testing and calibration.
- Compliance assurance: The accreditation ensures adherence to safety requirements, regulatory compliance, and customer satisfaction.
- Performance guideline: ISO 17025 promotes regular competency and data quality, ensuring accurate and reliable results.
- Increased business: The accreditation improves the brand image, increases client confidence, and provides a competitive advantage for international opportunities.
- Time and cost savings: Eliminates retesting, reduces the need for supplier audits, and enhances operational efficiency.
- Global business connections: ISO 17025 facilitates international trade and opens doors for new partnerships and collaborations.
- Reduced customer complaints: Adherence to ISO 17025 standards minimizes errors and improves customer satisfaction.
- Competitive edge: Laboratories with ISO 17025 accreditation gain a significant advantage over competitors.
- Technology development and usage: ISO 17025 encourages the adoption of new technologies for improved testing procedures.
- Proper equipment maintenance: The standard promotes the maintenance and calibration of test equipment for accurate and reliable results.
ELIGIBILITY FOR ISO 17025:2017 CERTIFICATION
Obtaining ISO 17025 certification does not have any specific eligibility requirements. The standard applies to all organizations performing laboratory activities, regardless of size, ownership, or sector. Whether you are a government laboratory, a private commercial lab, a research institution, or any other organization involved in testing, calibration, or sampling, you can pursue ISO 17025 certification.
The focus of ISO 17025 is on the competence, impartiality, and consistent operation of laboratories. To obtain the certification, organizations must demonstrate compliance with the requirements specified in the standard. It includes technical expertise, qualified personnel, appropriate equipment, suitable facilities, documented procedures, quality control measures, and a commitment to continuous improvement.
Finally, any organization dedicated to quality, accuracy, and reliability in its laboratory activities can strive for ISO 17025 certification.
THE COST OF ISO 17025:2017 CERTIFICATION
The Cost of ISO 17025 certification can vary significantly based on several factors. These include the laboratory’s location, size, accreditation scope, and infrastructure. Laboratories should be prepared for a substantial investment, potentially amounting to hundreds of thousands of dollars. However, cost-sharing with other accredited sections or programs can help mitigate expenses. Initial costs include a one-time purchase of software and equipment like LIMS (Laboratory Information Management System) and document control systems, while annual maintenance fees are relatively lower. Location impacts costs, including salaries for quality assurance staff and travel expenses for auditors. Existing policies, procedures, equipment, and software also influence certification costs, and laboratories with a robust quality management system in place may reduce expenses. It’s important to note that achieving certification may introduce additional fees, such as obtaining calibration services from ISO 17025-accredited providers.
CHALLENGES AND SOLUTIONS IN ISO 17025:2017 CERTIFICATION
CHALLENGES IN ISO 17025 CERTIFICATION
- Resource Constraints: Limited budget and inadequate resources for implementing ISO 17025 requirements
- Lack of Awareness and Understanding: Insufficient knowledge about ISO 17025 standards and requirements
- Documentation and Documentation Control: Difficulties in Establishing and Maintaining Documentation Systems
- Process standardization: Varying processes across different departments or locations
- Competence and Training: Ensuring staff competency and providing adequate training opportunities
- Nonconformities and Corrective Actions: Addressing nonconformities and implementing effective corrective actions
- Risk Management: Identifying and managing risks associated with laboratory processes and activities
- Impartiality and Conflicts of Interest: Ensuring impartiality and managing potential conflicts of interest
- Continuous Improvement: Sustaining a culture of continuous improvement and meeting evolving customer needs
- Post-Certification Maintenance: Ensuring ongoing compliance and maintaining the ISO 17025 certification
SOLUTIONS IN ISO 17025:2017 CERTIFICATION
- Prioritize resource allocation, seek external funding or partnerships, and optimize resource utilization through efficient planning.
- Conduct training and awareness programs for staff, engage consultants or experts for guidance, and promote continuous learning and education.
- Develop clear documentation procedures, implement document control mechanisms, and use software tools for efficient document management.
- Identify and streamline processes, establish standardized procedures, and encourage knowledge sharing and collaboration among teams.
- Conduct competency assessments, provide training programs, encourage professional development, and establish a culture of continuous learning.
- Conduct regular internal audits, establish a robust nonconformity management system, and prioritize timely corrective actions.
- Implement a risk assessment framework, establish risk mitigation strategies, and regularly review and update risk management plans.
- Establish clear policies and procedures for maintaining impartiality, implement conflict of interest management mechanisms, and promote transparency.
- Encourage feedback and suggestions from customers, establish performance metrics, conduct regular reviews, and implement improvement initiatives.
- Conduct regular internal audits and management reviews, implement a robust quality management system, and actively participate in proficiency testing programs.
DURATION OF ISO 17025:2017 CERTIFICATION
The duration of ISO 17025 certification varies depending on several factors. The initial certification process typically takes several months to a year. It includes preparing documentation, implementing the necessary quality management system, conducting internal audits, and addressing non-conformities. Once the certification body completes the on-site assessment and verifies compliance, the laboratory receives the ISO 17025 certification. The certification is valid for a specific period, usually three years, during which the laboratory must maintain compliance and undergo regular surveillance audits by the certification body. After the expiration of the certification, the laboratory can apply for recertification by demonstrating continued adherence to the standard’s requirements.
CERTPRO’S ASSISTANCE IN ACHIEVING ISO 17025:2017 CERTIFICATION FOR YOUR BUSINESS
CertPro specializes in assisting businesses to achieve ISO 17025 certification through their comprehensive auditing, consulting, and certification services. With a team of experienced auditors and consultants, CertPro guides your business through the certification process, adhering to industry best practices and international standards. They assess your laboratory management system, ensure compliance with ISO 17025 requirements, and help implement effective quality control measures. CertPro provides documentation support, training, and expert guidance to enhance the accuracy and reliability of your testing and calibration processes. By partnering with CertPro, your business can demonstrate competence and reliability in laboratory operations, gain recognition in the industry, and meet customer expectations. Achieving ISO 17025 certification with CertPro’s assistance establishes your commitment to quality, accuracy, and continuous improvement in laboratory services.
FAQ’s
HOW LONG DOES IT TAKE TO GET ISO 17025 CERTIFIED?
The timeframe for ISO 17025 certification depends on the laboratory’s readiness and the certification body’s process. It typically takes several months to a year to complete the certification process.
WHAT ARE THE CHANGE IN ISO 17025:2017?
ISO 17025:2017 introduced several changes, such as replacing Management requirements and Technical Requirements with General Requirements and Structural Requirements. It also added clauses for Resource Requirements and Process Requirements, placing greater emphasis on laboratory competence and impartiality.
WHAT IS THE CONSEQUENCE OF ISO 17025 NON-COMPLIANCE?
Non-compliance with ISO 17025, the international standard for testing and calibration laboratories, can result in various consequences that impact the reputation, operations, and legal standing of a laboratory.
WHAT IS THE SIGNIFICANCE OF ISO 17025 ACCREDITATION FOR A LABORATORY?
ISO 17025 accreditation enhances a laboratory’s reputation, ensures compliance with quality standards, promotes reliable testing and calibration, increases customer confidence, and opens doors to international business opportunities. It also helps in improving operational efficiency and reducing errors.
CAN ISO 17025 CERTIFICATION BE REVOKED?
Yes, ISO 17025 certification can be revoked if a laboratory fails to maintain compliance with the standard or address identified non-conformities. Regular surveillance audits and re-certification assessments are conducted to ensure continued adherence to the requirements.
UNDERSTANDING ISO 42001: A GUIDE FOR RESPONSIBLE AI MANAGEMENT SYSTEMS
The invention of artificial intelligence (AI) has changed the operational processes of many industries. However, the rapid growth of technology increases ethical, security, and privacy-related concerns. Therefore, the International Organization for Standardization...
EUROPEAN UNION’S ARTIFICIAL INTELLIGENCE ACT: HOW THIS GROUNDBREAKING LAW AFFECTS YOUR BUSINESS
Nowadays, Artificial Intelligence (AI) is transforming our lives exceptionally well. AI is now streamlining healthcare services, providing virtual assistance, and fulfilling queries. Technologies have boons and curses. Similarly, AI creates many concerns about...
How to Implement GRC Frameworks in 2024: Step-by-Step Guide
The rapidly evolving business environment, complexity, and accountability enhance the importance of the organization's governance, risk management, and compliance initiatives. Therefore, if your company finds difficulties expanding, recheck your organization's...